How Many Valence Electrons Does Molybdenum Have
Molybdenum-97 is composed of 42 protons 55 neutrons and 42 electrons. Valence electrons in Ruthenium Ru 8.

Molybdenum Mo Electron Configuration And Orbital Diagram
157 views View 1 share Related Answer.
. Molybdenum-96 is composed of 42 protons 54 neutrons and 42 electrons. How many unpaired electrons does manganese have Posted on January 1 2022 by admin Frequently asked questions. This is really a tough question to answer because of the ambiguity involved.
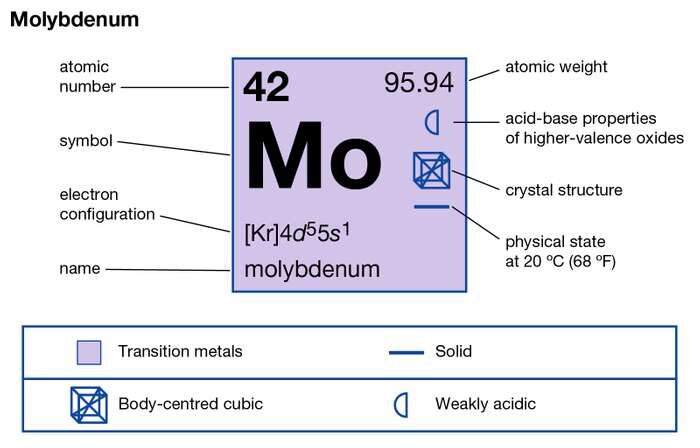
Schematic electronic configuration of molybdenum. 5s1 and the term symbol is 7S3. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus.
It depends it can either have valence electrons of 65432. Molybdenum atoms have 42 electrons and the electronic shell structure is 2 8 18 13 1. If in the teens drop the 1.
Each electron is influenced by the electric fields produced by the positive nuclear charge and the other Z 1 negative electrons in the atom. The name is from Neo-Latin molybdaenum which is based on Ancient Greek Μόλυβδος molybdos meaning lead since its ores were confused with lead ores. Valence electrons in Rhodium Rh 9.
42 Mo Available molybdenum properties. Molybdenum valence electrons If in the teens drop the 1. How many electrons does Mo have.
The electrons in the last shell is equal to sulfur S valence electrons. Molybdenum is a chemical element with atomic number 42 which means there are 42 protons and. Therefore the number of electrons in neutral atom of Molybdenum is 42.
13 Allrod Rochow Heat of Fusion. 42 electrons 42 protons and 54 neutrons. Molybdenum Overview Molybdenum Valence Electrons 236 Atomic Number 42.
The atomic number of molybdenum is 42 and its electron configuration is 1s22s22p63s23p63d104s24p64d55s1 or 2 8 18 13 1 electrons per shell. So this element is in group 16 so it. Molybdenum has six valence electrons.
Molybdenum have Atomic number of 42 The electronic configuration of molybdenum 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d5 5s1 The electrons in the 4d55s1 constitute its valence electrons 5 1 6 Therefore Molybdenum has six valence electrons. 6Molybdenum is within group 16. Molybdenum minerals have been known throughout history but the element was discovered.
Please help H. The electron configuration is Kr4d 5 5s 1 It is true that Mo has other valance states from -2 to 6 but it has six electrons AVAILABLE for bonding and there are many compounds in which Mo has an oxidation state of 6. How many valence electrons does molybdenum have.
There is an easy path to find valence electron which are located in the last shell of Sulfur. A valence electron is an outer shell electron and may participate in the formation of a chemical bond. Copper Cu and Silver Ag T or F.
For atoms with many electrons this notation can become lengthy and so an abbreviated notation is usedThis is important as it is the Valence electrons 4d5 5s1 electrons in the outermost shell that determine the chemical properties of the element. Science the question says assume the valencies of hydrogen nitrogen and carbon in the compounds are 1 3 and 4 from this i must draw a structural formulae for hydrogen cyanide and cyanogen that are consistant with these valencies. How many protons neutrons and electrons does Molybdenum have.
Chemical Properties of Molybdenum. The neutral atom of molybdenum has 42 electrons. It means that Sulfur atom has 16 protons and 16 electrons.
Molybdenum-95 is composed of 42 protons 53 neutrons and 42 electrons. Valence electrons in Technetium Tc 7. Now lets check the facts about Molybdenum.
Valence Electron Potential -eV. For main group elements ie s-block and p-block elements the valence electrons are the electrons present in the outermost orbit. The electrons in the 4d55s1 constitute its valence electrons.
How many valence electrons does molybdenum have. Valence electrons in Molybdenum Mo 6. How many valence electrons does molybdenum have.
The symbol for molybdenum is Mo. Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. Molybdenum-94 is composed of 42 protons 52 neutrons and 42 electrons.
4d 5 5s 1 Electron Dot Model. Molybdenum atoms have 42 electrons and the shell structure is 2818131. 6 valence electrons 2.
The easy way is to write the electron configuration. In the case of Molybdenum the valence electrons is 236. So Sulfur valence electrons are six.
The ground state electron configuration of ground state gaseous neutral molybdenum is Kr. 157 views View 1 share Related Answer Benjohn Barla. View Exam 1 Study Guidedocx from MET 14300 at Purdue University.
If in group 1 then it has 1 if group 2 then it has 2. Ok but how many valence electrons does an atom of Molybdenum have. Molybdenum have Atomic number of 42 The electronic configuration of molybdenum 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d5 5s1 The electrons in the 4d55s1 constitute its valence electrons 5 1 6 Therefore Molybdenum has six valence electrons.

Mo Molybdenum Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids

Molybdenum Atomic Structure Stock Image C018 3723 Science Photo Library

Molybdenum Valence Electrons Molybdenum Valency Mo Dot Diagram

2022 Valence Electrons In Molybdenum Mo Facts Color Discovery
No comments for "How Many Valence Electrons Does Molybdenum Have"
Post a Comment